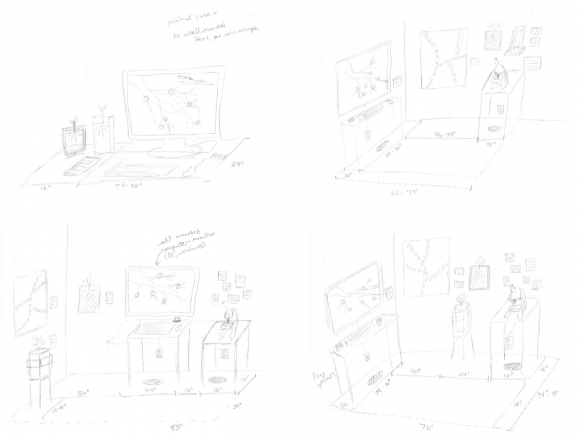
I wrote about some initial exhibition setup about a month ago, when we first started thinking about the thesis exhibition design. Since then, we’ve been developing these ideas a bit further, and testing out different approaches that we could take with the space. The biggest challenge for my project is finding ways to make it extend out of the digital space into the exhibition gallery. At this point, it seems unlikely that the thesis website will even be a part of the exhibit itself; we’re being asked to use video and other means to summarize and give viewers a flavor of the project without asking them to actually engage with the work itself.
My simplest sketches started out with two plants; one in soil, one in soil substitute, as a way of attracting attention to the core issue of what a world without soil might look like. Paired with a video overview of the site content, this could work as a quick introduction.
For our initial sketches, we were asked to dream big, as if space and equipment were no issue. In that case, I thought I might consider having a microscope with soil samples available, to allow users to actually see some of the bustling life in the soil. Adding photos or a poster with additional information could help to round out the experience, while also providing some breathing space between the long dwell-time displays (the microscope and the screen).
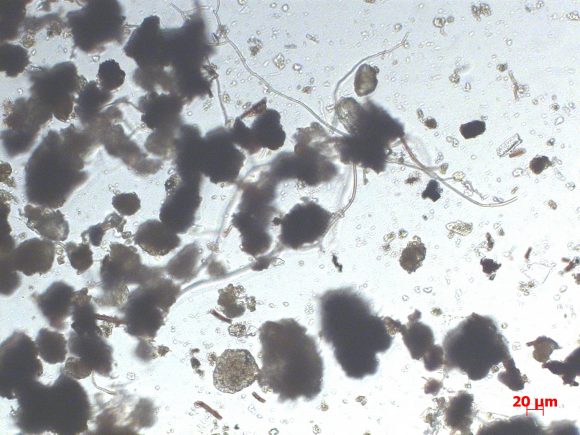
I’ve never tried looking at soil samples under the microscope, so it seemed like a good idea to start testing sooner rather than later. Branden has a microscope at work, and I’ve made a couple of trips in to look at samples from my garden and from a forest where we like to go for walks. I was really excited to see what we could find, but it turns out that there isn’t all that much going on in the soil in January. (I really could have picked a better topic for winter projects.)
We were able to see soil particles, and lots of plant root hairs.
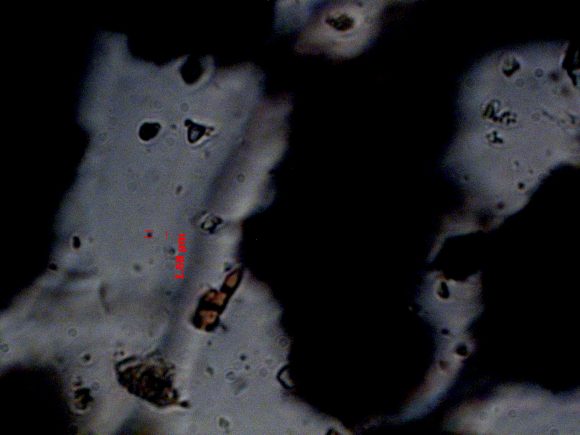
At higher magnification, I was also able to make out what I think are protozoa swimming around (there’s one in between the red lines on the length scale).
Bacteria would be even smaller than this, but I’d have expected to see other larger organisms as well, if the sample were taken at a more active time of year. Nematodes, tardigrades and rotifers are on the mm length scale, but I didn’t find any of those in my samples. I’m not sure whether it’s just because they migrate below the frost line in the winter, or whether I sampled in the wrong locations or prepped the microscope slides badly. I’m still interested in considering this as an option, but it seems good to have a backup plan, too.
I was looking at the soil samples again tonight, and noticed a great example of mycorhizzal fungi growing around this plant root (click on the picture to enlarge). It looks like a fine white spider web, but it’s made of tiny threads of fungi that allow plant roots to communicate and pass food to one another, and also serve as “bacterial highways” in the soil. I was able to get a pretty good photo of it with our macro lens, but it would be even better under a microscope.
I’ve also been checking in on the plant cuttings that I made for the exhibition a few weeks ago. I was concerned that they might not make it. Cuttings are delicate things, and very vulnerable to mold and fungal infections or dehydration before their roots form, so it seemed better to hedge my bets and get a few plants started now, in case the first ones don’t survive.
The framed cutting is looking a little sad; it lost several of its leaves in the first week or so.

But it also has some new ones starting, so I think it’s probably going to be ok.

The soil substitute cutting is looking even worse for wear; I thought for sure I was going to lose it when I found mold growing on its stem last week. But a few days in direct sun seems to have cleared that up, and it also has hints of new growth and maybe even a few roots starting to form.



This little one was never even meant to be a cutting; it was just big enough that I felt badly throwing it out, so I stuck it in a jar of water and ignored it. Of course, it has the best root of the lot, so far. I transferred it into the soil substitute last week, and it seems to be doing just fine.
I made a few soil samples in regular pots, partly because the tree needed to be cut back and partly to have a couple more spares, just in case. One of them is very unhappy as of yesterday, but the other seems to be doing just fine.

I also made some cuttings of an umbrella tree, which has also wildly outgrown its pot. I thought it might be helpful to have a second kind of plant, in case one grows roots faster than another (I know lots about plants, but realized that I really have no idea how fast their roots grow, since they’re always hidden in the dirt. Even the gardener at my local greenhouse didn’t have a good guess…apparently, it’s not something that you really think about, even when you grow plants for a living.)

I am hopeful about this one, though. The cutting is two weeks younger than the others, and it already has some pretty good root buds forming along the stem.
So, while I work in my office, I am surrounded by an army of little plants, wrapped in aluminum foil to protect their roots from the light. Hopefully at least a couple will be in good shape for the exhibition, and then I’m going to have a lot of houseplants to give away!